4 Questions On NAD/NADH Testing Answered
Unlocking the Secrets of Cellular Energy
5 min read
![]() Dr. Andrea Gruszecki, ND
:
November 1, 2021 at 2:37 PM
Dr. Andrea Gruszecki, ND
:
November 1, 2021 at 2:37 PM

As winter approaches, monitoring the antibody status of patients who have been vaccinated or exposed to the COVID-19 virus is imperative to patients’ health. Luckily, multiple studies have been completed providing guidance as to which testing option is the superior choice.
As the holiday season approaches those of us who will be traveling and visiting distant friends or relatives may want information about their immune status to COVID-19. While a large segment of the population may have had an asymptomatic COVID-19 infection, an even larger segment of the population believes that they have had a COVID-19 infection without a proof (“thinkihadititis”). Depending upon how long ago those COVID-19 exposures were, their virus-induced antibody levels may have declined. In such cases, these individuals may contract a fresh COVID-19 infection and inadvertently expose fellow travelers or loved ones.
For the unvaccinated, a new study indicates that re-infection with COVID-19 can be expected on average 16 months after antibodies peak from a prior COVID-19 infection, and as early as 3 months after the antibody peak in some individuals.
Since proof of immunity is now often a requirement for travel or large public events, and there may be no reason to vaccinate if natural immunity is present, there has been an increased interest in proving past COVID-19 infection. Unfortunately, the nature of the immune response against the COVID-19 virus makes the detection of past COVID-19 infections difficult.
While there has been much interest in T-cell testing for past COVID-19 infections, side-by-side testing for T-cells and COVID-19 “neutralizing” antibodies such as the Spike (S-RBD protein) antibody clearly demonstrates the superiority of antibody testing, especially in milder or asymptomatic COVID-19 infections where T-cell responses are more variable. In addition, some T-cells show high rates of cross-reactivity with non-COVID-19 viruses. T-cell assessments cannot determine whether or not protective “neutralizing” antibodies are being produced. Finally, one popular T-cell test claims that T-cell COVID-19 responses can be detected for years, yet a close look at the references cited shows only an article about multi-year responses for severe acute respiratory syndrome (SARS), not the COVID-19 virus.
Antibodies are produced by B-cells. After antigen exposure (by virus or vaccine) and recovery, low levels of pathogen-specific antibodies are continuously produced by long-lived plasma B-cells in the bone marrow, providing serological memory for years after the pathogen has been cleared. If reinfection occurs, these antigen-specific memory B cells can quickly produce new antibodies.
Not every patient is able to mount an appropriate antibody response to a COVID-19 challenge (virus or vaccine) because immune responses are complicated and require the interaction of many different cell types (e.g., B and T cells, NK cells, macrophages, etc.). In addition, physical barriers in the lungs and gut may or may not be effective in some individuals. The immune system and barrier functions are also influenced by medications, comorbid inflammatory disorders, stress, mitochondrial function, and age.
T-cells can encourage B-cells to make antibodies, but they are not necessary for effective viral pathogen responses. A study in macaque monkeys demonstrated that, if T-cells were removed, the monkeys could recover from COVID-19 infection, and that that the T-cell deficient monkeys were more efficient at eliminating a deliberate COVID-19 re-infection 6 weeks later!
Human studies that measure both COVID-19 antibodies and T-cell responses clearly demonstrate the utility of Spike antibody assessment:
US BioTek offers several options for COVID-19 antibody testing. The Immune Response Panel, which can discern the source of antibody inoculation (virus vs vaccination) is the panel with the most clinical utility for current circumstances. The Immune Response panel tests for Nucleocapsid (N) antibodies. N antibodies are created in response to the COVID-19 virus only, not from vaccination. The Spike antibody (S-RBD) antibodies can occur from either vaccination or COVID-19 exposure.
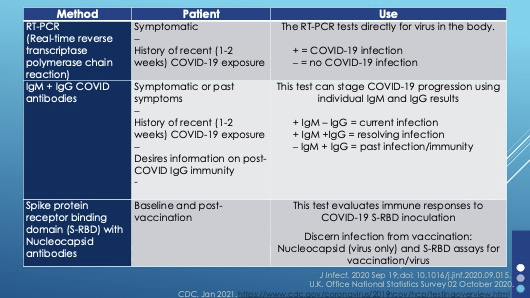
The Immune Response panel can be used for both a pre-vaccination baseline or in series to track the production of antibodies from a COVID-19 exposure. Because the test combines short-lived IgM and long-lived IgG antibodies together in a single result, the Immune Response panel cannot be used to monitor the stages of a COVID-19 infection.
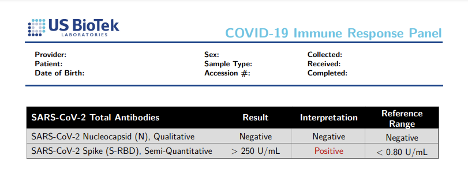
In contrast, the IgG+IgM Antibody panel can be used to stage a patient’s response to COVID-19 infection, however, because this panel measures a combination of S1, S2, and N antibodies it cannot be used to monitor the patient’s response to vaccination.
Since so many COVID-19 infections have been asymptomatic, it is impossible to tell if Spike antibodies are increasing or decreasing without serial testing. Testing at least twice, several months apart will clearly demonstrate whether antibody levels are increasing, stable or decreasing.
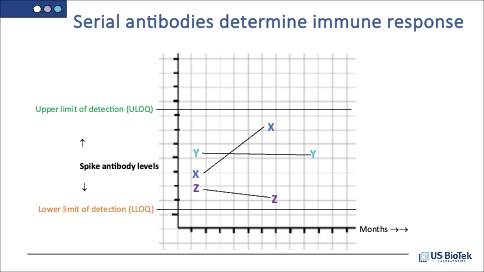
In the example above, patient X is clearly still increasing antibody levels, patient Y has stable antibody levels, and patient Z has declining levels. If indicated, it may be time to discuss vaccination or booster shots with patient Z.
The choice seems to be clear – Spike (S-RBD) antibody testing is superior to T-cell tests and is the better choice for both distant and recent COVID-19 infection or vaccination status. Serial antibody testing indicates whether antibody levels are increasing, stable, or decreasing. As winter approaches, clinicians need to be able to monitor the antibody status of patients who have been vaccinated or exposed to the COVID-19 virus.
Learn about the different types of COVID tests available for your practice:
Castro Dopico X, Ols S, Loré K, Karlsson Hedestam GB. Immunity to SARS-CoV-2 induced by infection or vaccination. J Intern Med. 2021 Aug 5:10.1111/joim.13372.
Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021 Feb 5;371(6529):eabf4063.
Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020 Jun 25;181(7):1489-1501.e15.
Hasenkrug KJ, Feldmann F, Myers L, Santiago ML, Guo K, Barrett BS, et al. Recovery from acute SARS-CoV-2 infection and development of anamnestic immune responses in T cell-depleted rhesus macaques. bioRxiv [Preprint]. 2021 Apr 4:2021.04.02.438262.
Judkis, M. (2020) So many people are convinced that they had COVID-19 already. https://www.washingtonpost.com/lifestyle/style/why-everyone-you-know-is-convinced-that-they-had-covid-19-already/2020/05/05/aef406ac-8a38-11ea-8ac1-bfb250876b7a_story.html Accessed 25 October 2021. Washington Post. Accessed 25 October 2021.
Reynolds CJ, Swadling L, Gibbons JM, Pade C, Jensen MP, et al. Discordant neutralizing antibody and T cell responses in asymptomatic and mild SARS-CoV-2 infection. Sci Immunol. 2020 Dec 23;5(54):eabf3698.
Townsend JP, et al. The durability of immunity against reinfection by SARS-CoV-2: a comparative evolutionary study. Lancet. 2021 Oct; DOI: https://doi.org/10.1016/S2666-5247(21)00219-6 . Accessed 26 October 2021.
Yao L, Wang GL, Shen Y, Wang ZY, Zhan BD, et al. Persistence of Antibody and Cellular Immune Responses in Coronavirus Disease 2019 Patients Over Nine Months After Infection. J Infect Dis. 2021 Aug 16;224(4):586-594.

Unlocking the Secrets of Cellular Energy
Short chain fatty acids (SCFAs) are organic acids produced by bacterial fermentation of dietary fibre and resistant starch. Enterocytes and...

Zonulin has emerged as a popular marker to assess the integrity of the intestinal mucosal barrier. Discovered by Dr Alessio Fasano, Zonulin...