4 Questions on NAD/NADH Testing Answered
Unlocking the Secrets of Cellular Energy
8 min read
![]() Dr. Andrea Gruszecki, ND
:
March 13, 2024 at 2:35 PM
Dr. Andrea Gruszecki, ND
:
March 13, 2024 at 2:35 PM

Why do some children have problems with IgE allergy as infants? A history of IgE-mediated allergic disorders in infancy or early childhood predisposes the child to a higher risk of severe allergy in adulthood. The mitigation or prevention of early allergy, as a means of reducing adult allergy risk, must be considered by pediatric and obstetric clinicians.
Infant allergy most often presents as atopic dermatitis, gastrointestinal symptoms, and/or recurrent wheezing. Infant symptoms usually begin as the result of an exposure to common food antigens and then progress to include indoor inhalant antigens. Older children can present with different symptoms such as bronchial asthma and/or allergic rhinoconjunctivitis (nasal congestion and red, watery eyes); these symptoms may be the result of food or inhalant exposures. Atopic disease (allergy, asthma, eczema) is induced when IgE sensitization takes place. Typically, the first antigen exposure is non-reactive but primes the immune system with a strong inflammatory allergic reaction to subsequent antigen exposures.
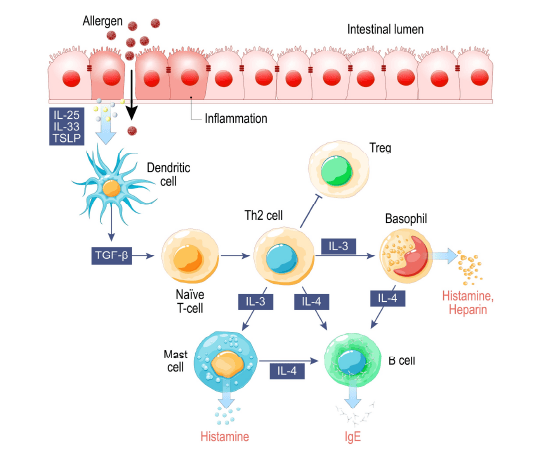
The risk of allergy, at any age, is increased when immune tolerance has been lost. The thymus gland, the cellular mitochondria, and the gut all work together to maintain tolerance. The thymus gland produces tolerant, anti-inflammatory T-regulatory cells (Tregs) and other lymphocytes essential to normal immune function. The mitochondria produce cell signaling molecules that promote tolerance or inflammation in the immune system, the thymus gland, and the gut mucosal lining. The mucosal lining of the gastrointestinal system regulates the amount and molecular size of antigens entering the body; a loss of gut barrier function is pro-inflammatory. An increased number of larger, foreign proteins (antigens) in circulation triggers the inflammatory immune response, and the activation of B-cells during inflammation increases the risk of IgE antigen sensitization and allergic responses. The gut microbiome “cross-talks” with the gut mucosa, and a diverse gut microbiome is tolerant and anti-inflammatory. Once the gut becomes chronically inflamed, there may be inflammatory shifts in the types of bacteria found in the gut microbiome.
The modern inflammatory “Western” lifestyle sets the stage for the induction of atopic diseases (allergy, asthma, eczema). Poor antioxidant status, gastrointestinal dysfunction, loss of microbial diversity, toxic exposures, pre-existing comorbid inflammation, poor nutrition and psychological stress can have a cumulative and synergistic effect that increases oxidative stress and chronic inflammatory diseases. Inflammation decreases mitochondrial function and damages mitochondria, which send out pro-inflammatory distress signals. The thymus responds by producing more aggressive pro-inflammatory Tregs and lymphocytes. Damaged mitochondria produce less ATP which affects the health and function of the both the thymus and gut mucosa. A compromised gut mucosa promotes barrier dysfunction and inflammatory cross-talk with the gut microbiome. The local inflammation in the gut mucosa affects microbiome diversity in the gastrointestinal environment and can permit the expansion of dysbiotic bacteria or true gut pathogens. Given the increasing prevalence of Western lifestyle risk factors around the globe, is it any wonder that the prevalence of infant/childhood allergy has roughly doubled worldwide in the 21st century?
Tolerance, Allergy, And Sensitivity
|
Studies indicate that, in addition to genetics and the infant’s external environment, the fetal maternal environment may influence the development of atopic disorders in utero. Contributing factors include maternal infection, maternal inflammation, maternal chemical exposure, and maternal allergy status. Prenatal mold exposure has been associated with an increased risk of eczema and higher total IgE levels at one year of age. The good news is that animal and human studies provide clues that may help the astute clinician “allergy-proof” a baby-to-be.
Family matters: Genetic and epigenetic influencesy
|
As with so many things, an ounce of prevention is worth a pound of cure. The timing of food introductions, especially of likely allergens such as egg and peanut, at about 6 months of age in a healthy infant has been shown to reduce the risk of allergy development. Conversely, maternal avoidance of likely allergens in the third trimester, the use of hydrolysated antigen-free formula or breast milk, and later infant food introduction of those likely allergens (dairy, egg, peanut) reduced the incidence of infant allergy in the first 2 years but did not alter later childhood risk at 7 years of age. In this study, childhood allergy risk was greatly influenced by gender and other environmental factors, such as household smoking, maternal ethnicity, and maternal asthma status. Therefore, the timing of food introductions is a clinical decision based upon the individual maternal lifestyle, medical history, and home environment.
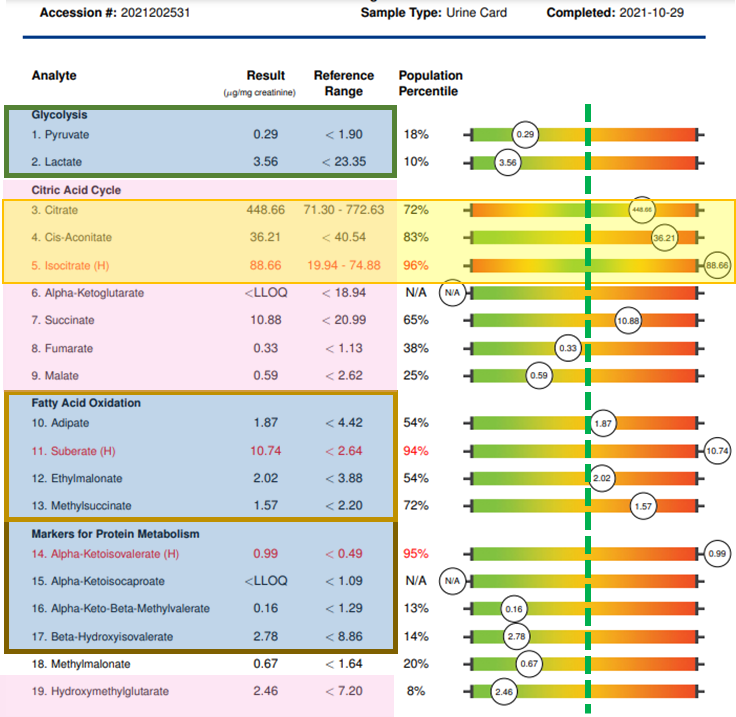
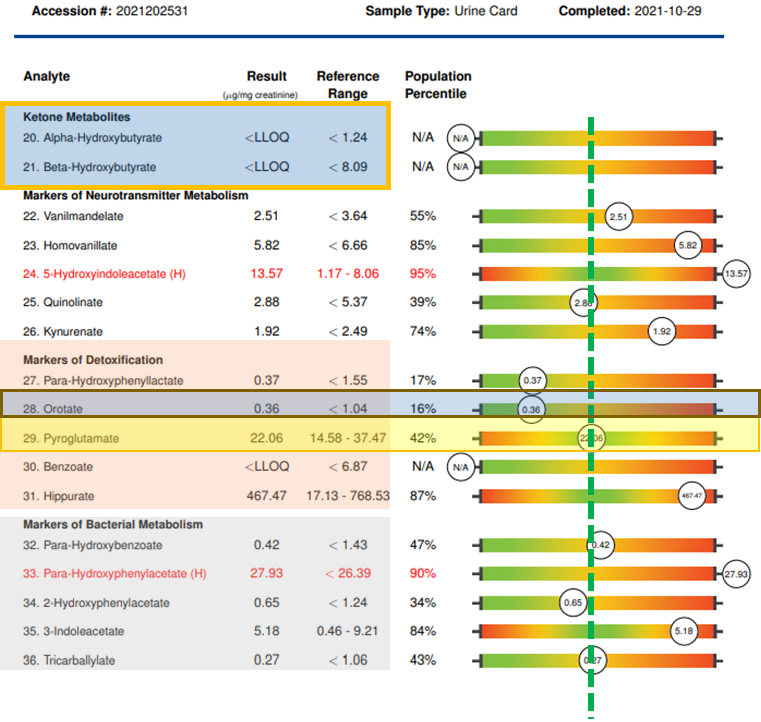
However, many environmental risk factors are avoidable or can be mitigated with lifestyle changes, antioxidants, and nutritional support. Pre-conception is always the best time to start, but decreasing risk factors and improving maternal health can be beneficial at any time in the pregnancy and even after birth while the infant is breast-feeding. Action steps can include US BioTek testing:
If desired, the Organic Acids Profile, the Environmental Pollutants Profile and the Mycotoxin Profile can also be bundled together as US BioTek’s MOE-TOX Complete Profile which saves the patient time and expense and provides the clinician a more comprehensive overview of patient exposures and metabolic function. While a vigorous detoxification program is contraindicated during pregnancy and breast-feeding, nutritional support of antioxidant status and correction of metabolic and GI function, and restoration of gut microbiome diversity can support both mother and fetus through pregnancy and beyond. Visit US BioTek’s Educational Resources page for a deeper exploration of the information on the following slides. Once test results are in, support strategies may include:
Promote Immune Tolerance
|
Minimize food- inhalant cross-reactions
|
| Adjust diet ratios | Antioxidants | Mitochondrial cofactors |
Identify, eliminate, detoxify environmental exposures |
Correct digestion to support microbiome |
Identify and eliminate toxicants
|
Conclusion
The worldwide incidence of perinatal allergy or atopic disease has roughly doubled in the 21st century. Don’t let your patients be part of this trend! Evaluate patients pre-natally to reduce the risk of allergic induction in infants and young children. Evaluate the children themselves as early as possible to promote tolerance and further mitigate the risk of adult allergies or atopic disorders in your patient population.
References
Annesi-Maesano I, Fleddermann M, Hornef M, von Mutius E, Pabst O, Schaubeck M, Fiocchi A. Allergic diseases in infancy: I - Epidemiology and current interpretation. World Allergy Organ J. 2021 Nov 12;14(11):100591.
https://pubmed.ncbi.nlm.nih.gov/34820047/
Chiu CY, Huang YL, Tsai MH, Tu YL, Hua MC, Yao TC, Yeh KW, Huang JL. Sensitization to food and inhalant allergens in relation to atopic diseases in early childhood: a birth cohort study. PLoS One. 2014 Jul 17;9(7):e102809.
https://pubmed.ncbi.nlm.nih.gov/25033453/
Cook-Mills JM. Maternal influences over offspring allergic responses. Curr Allergy Asthma Rep. 2015 Feb;15(2):501.
https://pubmed.ncbi.nlm.nih.gov/25612797/
Elhage R, Kelly M, Goudin N, Megret J, Legrand A, Nemazanyy I, Patitucci C, Quellec V, Wai T, Hamaï A, Ezine S. Mitochondrial dynamics and metabolic regulation control T cell fate in the thymus. Front Immunol. 2024 Jan 15;14:1270268. https://pubmed.ncbi.nlm.nih.gov/38288115/
Ghozal M, Kadawathagedara M, Delvert R, Divaret-Chauveau A, Raherison C, et al. Prenatal dietary exposure to mixtures of chemicals is associated with allergy or respiratory diseases in children in the ELFE nationwide cohort. Environ Health. 2024 Jan 9;23(1):5. https://pubmed.ncbi.nlm.nih.gov/38195595/
Høst A, Halken S. Hypoallergenic formulas--when, to whom and how long: after more than 15 years we know the right indication! Allergy. 2004 Aug;59 Suppl 78:45-52. https://pubmed.ncbi.nlm.nih.gov/15245358/
Järvinen KM, Martin H, Oyoshi MK. Immunomodulatory effects of breast milk on food allergy. Ann Allergy Asthma Immunol. 2019 Aug;123(2):133-143. https://pubmed.ncbi.nlm.nih.gov/31048004/
Jiao L, Su CW, Cao T, Zheng S, Walker WA, Shi HN. Maternal Influences and Intervention Strategies on the Development of Food Allergy in Offspring. Front Immunol. 2022 Feb 23;13:817062.
https://pubmed.ncbi.nlm.nih.gov/35281070/
Kawano Y, Morikawa M, Watanabe M, Ohshiba A, Noma T, Odajima H. A study of the factors responsible for the development of allergic diseases in early life. Asian Pac J Allergy Immunol. 2005 Mar;23(1):1-6.
https://pubmed.ncbi.nlm.nih.gov/15997868/
Lee E, Choi KY, Kang MJ, Lee SY, Yoon J, Cho HJ, et al. Prenatal mold exposure is associated with development of atopic dermatitis in infants through allergic inflammation. J Pediatr (Rio J). 2020 Jan-Feb;96(1):125-131.
https://pubmed.ncbi.nlm.nih.gov/30243937/
Pierau M, Arra A, Brunner-Weinzierl MC. Preventing Atopic Diseases During Childhood - Early Exposure Matters. Front Immunol. 2021 Feb 25;12:617731. https://pubmed.ncbi.nlm.nih.gov/33717110/
Podlecka D, Jerzyńska J, Sanad K, Polańska K, Bobrowska-Korzeniowska M, Stelmach I, Brzozowska A. Micronutrients and the Risk of Allergic Diseases in School Children. Int J Environ Res Public Health. 2022 Sep 26;19(19):12187. https://pubmed.ncbi.nlm.nih.gov/36231487/
Thapa P, Farber DL. The Role of the Thymus in the Immune Response. Thorac Surg Clin. 2019 May;29(2):123-131.
https://pubmed.ncbi.nlm.nih.gov/30927993/
Trogen B, Jacobs S, Nowak-Wegrzyn A. Early Introduction of Allergenic Foods and the Prevention of Food Allergy. Nutrients. 2022 Jun 21;14(13):2565. https://pubmed.ncbi.nlm.nih.gov/35807745/
Turner PJ, Arasi S, Ballmer-Weber B, Baseggio Conrado A, Deschildre A, et al; Global Allergy, Asthma European Network (GA2LEN) Food Allergy Guideline Group. Risk factors for severe reactions in food allergy: Rapid evidence review with meta-analysis. Allergy. 2022 Sep;77(9):2634-2652.
https://pubmed.ncbi.nlm.nih.gov/35441718/
Vuillermin PJ, Macia L, Nanan R, Tang ML, Collier F, Brix S. The maternal microbiome during pregnancy and allergic disease in the offspring. Semin Immunopathol. 2017 Nov;39(6):669-675.
https://pubmed.ncbi.nlm.nih.gov/34310928/
Zeiger RS, Heller S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol. 1995 Jun;95(6):1179-90. https://pubmed.ncbi.nlm.nih.gov/7797786/

Unlocking the Secrets of Cellular Energy
Short chain fatty acids (SCFAs) are organic acids produced by bacterial fermentation of dietary fibre and resistant starch. Enterocytes and...

Zonulin has emerged as a popular marker to assess the integrity of the intestinal mucosal barrier. Discovered by Dr Alessio Fasano, Zonulin...