4 Questions On NAD/NADH Testing Answered
Unlocking the Secrets of Cellular Energy

The winter months and holiday season provide many opportunities for happy times and visits with friends and family. Unfortunately, the season also provides many opportunities to spread pathogens, such as COVID-19 variants, to others as we work, visit, shop, and celebrate our way through the holiday season into the New Year.
An increasing number of human and other studies continue to validate the existence of “long COVID” and these studies are beginning to unravel the physiological pathways disrupted by COVID viral infections. While long COVID was originally considered a problem for those with severe COVID infections, newer studies indicate that long COVID also occurs more frequently in those with multiple COVID infections and can even occur in those with mild COVID infections under the right circumstances. Long COVID risk factors include increased age, chronic inflammatory disorders, and comorbid disease and the most commonly reported long COVID symptom is fatigue.
The effects of COVID-19 on mitochondrial function, ROS production, and prolonged inflammation via COVID proteins such as ORF-9b, are now well documented and explain much of the perceived fatigue described by long COVID patients. COVID infection hijacks the mitochondrial antiviral signaling response and shuts down early innate immune responses to the virus. The altered signaling permits the survival of damaged mitochondria, which then continue to send chronic inflammatory signals out to the surrounding tissues and the immune system after the infection is over. Long COVID dysfunctions may affect any organ system, including the liver, heart, or nervous system. In general, organs with many mitochondria and higher metabolic rates are most affected by COVID cellular dysregulation. For those unfamiliar with COVID effects on mitochondrial function, and the clinical utility of dried urine Organic Acids testing in the assessment of mitochondrial function, additional resources available from US BioTek have been provided at the end of the article.
A new human study being prepared for peer-reviewed publication implicates chronic dysregulation of the complement signaling pathway as a contributing factor in long COVID. This pre-print study indicates that biomarkers of classical complement pathway activation are consistently elevated in long COVID patients (78% predictive power). Given the amount of dysregulation found in long COVID patients, decreasing inflammation by whatever means are available makes good clinical sense. Several human studies indicate that restoration of mitochondrial function via supplementation can improve recovery rates in mild-to-moderate COVID infection. Ideally, such anti-inflammatory strategies are monitored by pre-post testing. Modification of environmental inputs (diet, toxins and lifestyle) may be employed to help regulate the complement system and other inflammatory pathways that contribute to long COVID symptoms.
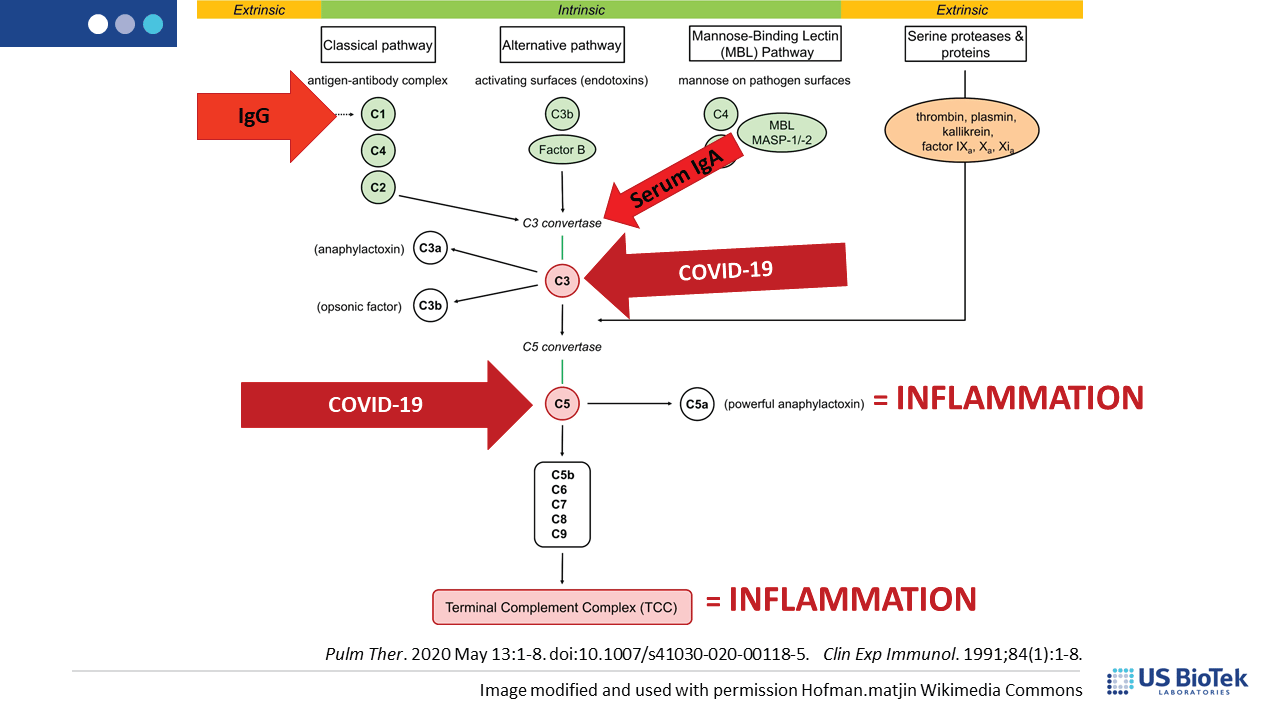
Complement is a group of circulating proteins that contribute to the activation of mast cells and antibody-producing B-cells during infection. Complement activation is expected during viral infections, and is facilitated by MAVS, damage-associated molecular patterns (DAMPs) or other inflammatory signaling. Unfortunately, COVID-induced immune system dysregulation may prevent the return of complement homeostasis post-infection, especially if there was pre-existing inflammation before COVID. Some complement proteins can directly affect mitochondrial function, decreasing the amount of cellular energy available for recovery. Dysfunctional or damaged mitochondria produce DAMPS that perpetuate inflammatory signaling, creating a vicious cycle of chronic post-COVID inflammation.
Complement dysregulation may occur for many reasons, many of which can be assessed and addressed by an astute clinician to reduce the level of inflammatory complement signaling overall. Reducing pressure on, and signaling from, the complement system can help restore normal immune function. Complement may activate in response to infection, cell damage or other inflammatory insults such as:
Assessment of mitochondrial and metabolic function, digestive function, toxin exposure and immune system responses to environmental antigens can uncover a variety of actionable items, including:
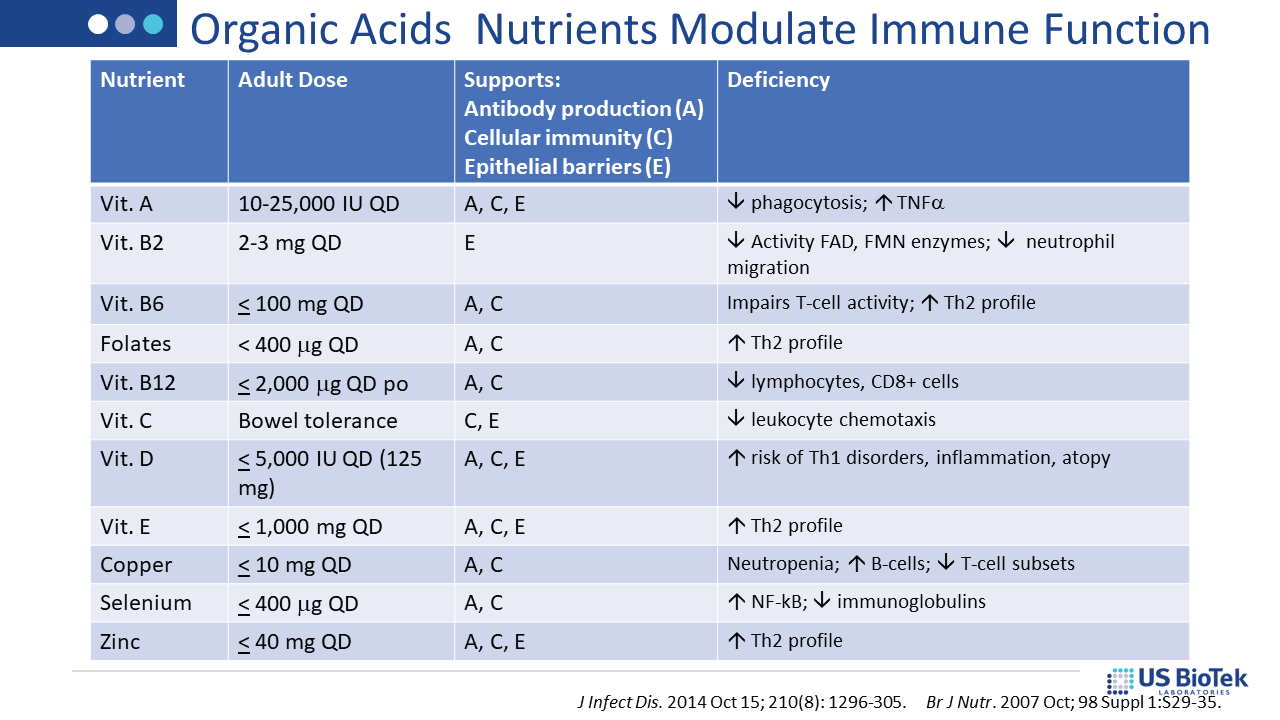
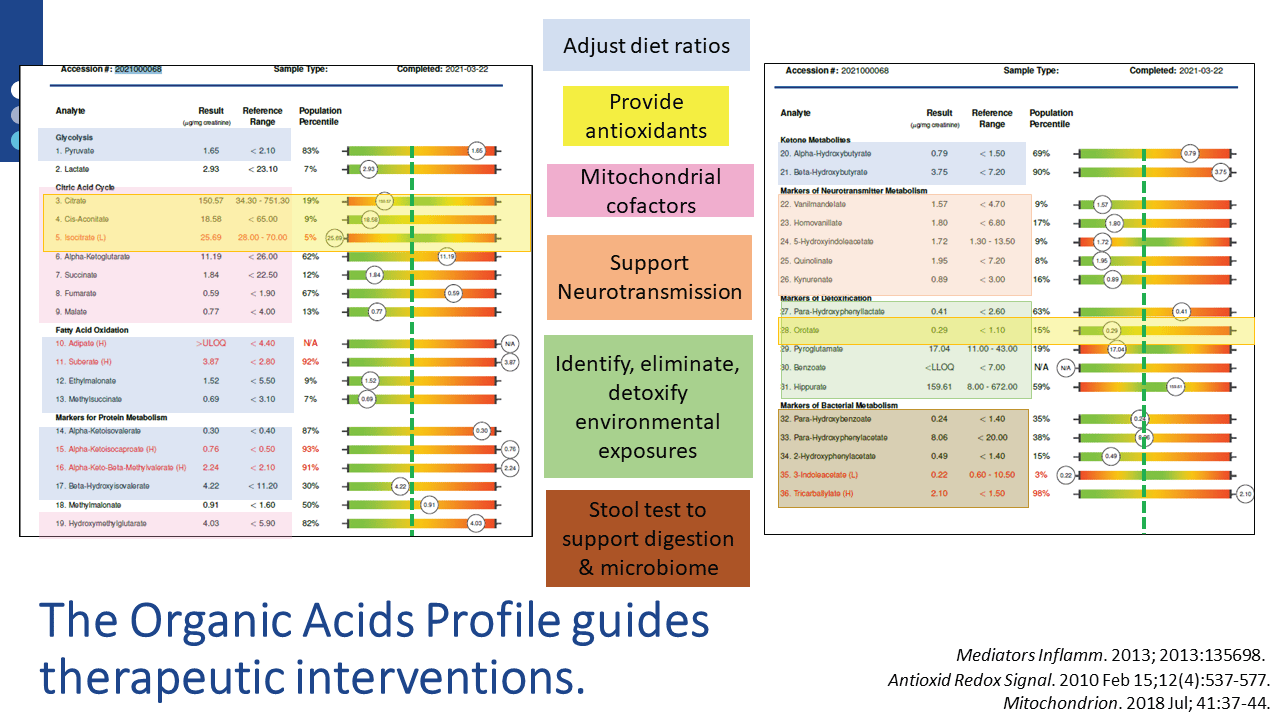
Once the assessment is completed, specific nutritional and lifestyle recommendations are available via US BioTek’s Interpretation Guides or live consultations. For example, nutrient supports indicated by a patient’s Organic Acids Profile (OAP) results are also supportive of homeostatic, tolerant immune function. OAP results can also guide a variety of other interventions, as shown below:
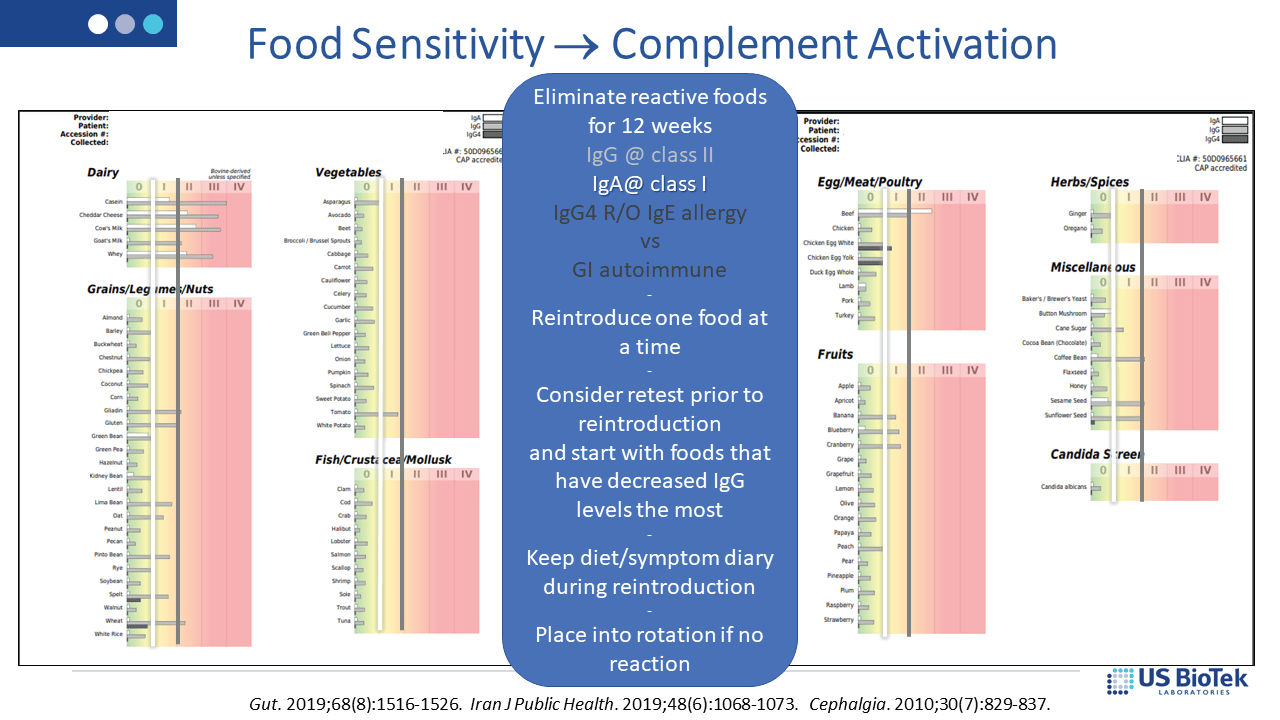
Dietary restriction based upon food sensitivity results (below) can decrease overall IgG and IgA antibody loads; lower antibody loads may then help regulate the complement system. IgE allergy is best treated with strict avoidance of the offending antigen – reintroduction is not recommended for known allergens and allergic responses increase histamine-driven inflammation.
Identification and elimination of toxicants (below), either mold or chemical, decrease detoxification burdens on the liver and may also have beneficial effects on mitochondrial and immune system functions. Liver detoxification status can be assessed on Organic Acids Profile results, which can be performed in tandem with either chemical or mycotoxin evaluations.
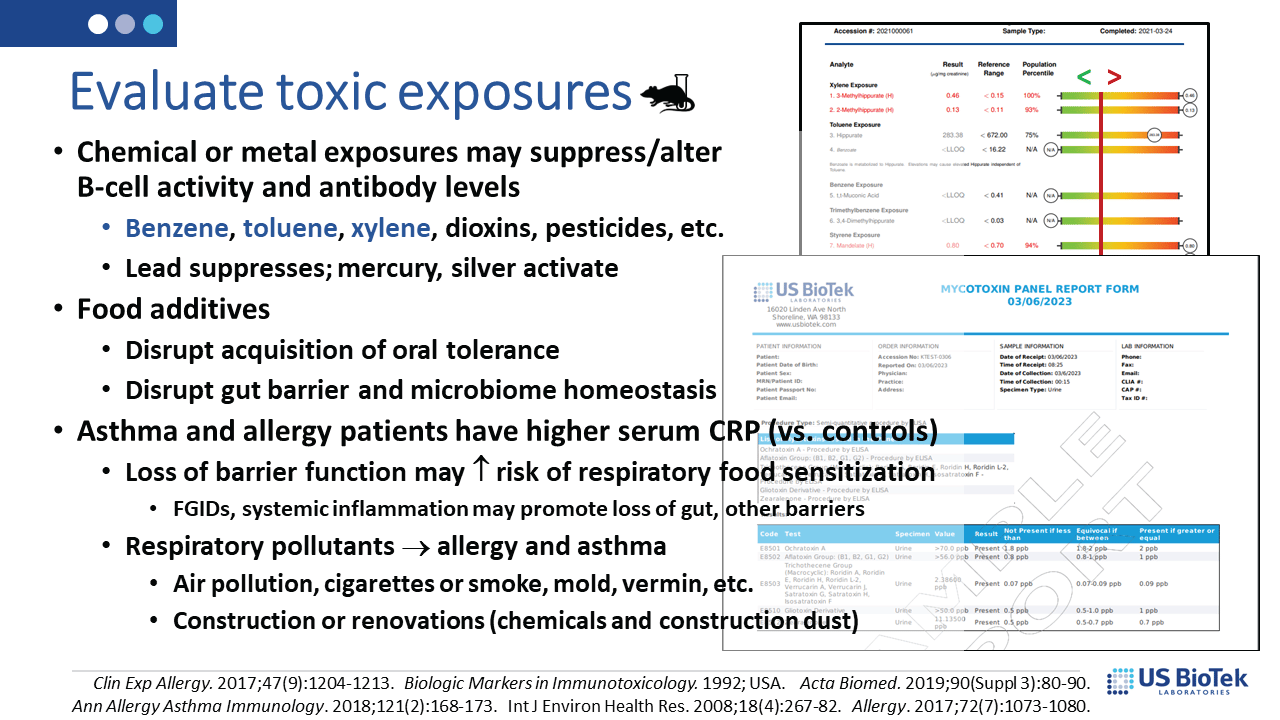
Lastly, the status of the gut microbiome and digestive functions (below) determine not only local, but systemic inflammation or environmental tolerance. The evaluation of digestive function and microbiome diversity is an essential step in reversing runaway inflammatory signaling.

After results are evaluated, lifestyle adjustments are typically included in the clinician’s treatment plan. Depending upon the test and it’s results, recommendations may include
While these interventions could prove useful for a long COVID patient, prevention is always the best choice. Improving overall health and antioxidant status, while decreasing a patient’s baseline inflammation load before a COVID infection could significantly decrease not only COVID symptom severity and duration, but also decrease the risk of long COVID.
COVID-19 in Multiple Organs—What's the Connection? >>> Click Here
OXIDATIVE STRESS, VIRAL INFECTION, AND MITOCHONDRIAL FUNCTION >>> Click Here
Organic Acids in Health and Disease >>> Click Here
CE Webinar: Long COVID-19: Unravelling the Neurological Consequences >>> Click Here
Ajaz S, McPhail MJ, Singh KK, Mujib S, Trovato FM, Napoli S, Agarwal K. Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. Am J Physiol Cell Physiol. 2021 Jan 1;320(1):C57-C65.
Altay O, Arif M, Li X, Yang H, Aydın M, Alkurt G, et al. Combined Metabolic Activators Accelerates Recovery in Mild-to-Moderate COVID-19. Adv Sci (Weinh). 2021 Sep;8(17):e2101222.
Baillie K, Davies H, Keat S, Ladell K, Miners K, et al. Complement dysregulation is a predictive and therapeutically amenable feature of long COVID. medRxiv (preprint) 2023 doi: https://doi.org/10.1101/2023.10.26.23297597 Accessed 19 December 2023.
Boufidou F, Medić S, Lampropoulou V, Siafakas N, Tsakris A, Anastassopoulou C. SARS-CoV-2 Reinfections and Long COVID in the Post-Omicron Phase of the Pandemic. Int J Mol Sci. 2023 Aug 19;24(16):12962.
Baroiu L, Dumitru C, Iancu A, Leșe AC, Drăgănescu M, Baroiu N, Anghel L. COVID-19 impact on the liver. World J Clin Cases. 2021 Jun 6;9(16):3814-3825.
Chen TH, Chang CJ, Hung PH. Possible Pathogenesis and Prevention of Long COVID: SARS-CoV-2-Induced Mitochondrial Disorder. Int J Mol Sci. 2023 Apr 28;24(9):8034.
Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023 Mar;21(3):133-146.
Guo Z, Fan X, Yao J, Tomlinson S, Yuan G, He S. The role of complement in nonalcoholic fatty liver disease. Front Immunol. 2022 Sep 29;13:1017467.
Perricone C, De Carolis C, Giacomelli R, Greco E, Cipriani P, Ballanti E, Novelli L, Perricone R. Inhibition of the complement system by glutathione: molecular mechanisms and potential therapeutic implications. Int J Immunopathol Pharmacol. 2011 Jan-Mar;24(1):63-8.
Refolo G, Vescovo T, Piacentini M, Fimia GM, Ciccosanti F. Mitochondrial Interactome: A Focus on Antiviral Signaling Pathways. Front Cell Dev Biol. 2020 Feb 14;8:8.
Wesselink E, Koekkoek WAC, Grefte S, Witkamp RF, van Zanten ARH. Feeding mitochondria: Potential role of nutritional components to improve critical illness convalescence. Clin Nutr. 2019 Jun;38(3):982-995.
West EE, Kemper C. Complosome - the intracellular complement system. Nat Rev Nephrol. 2023 Jul;19(7):426-439.
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010 Mar 4;464(7285):104-7.

Unlocking the Secrets of Cellular Energy
Short chain fatty acids (SCFAs) are organic acids produced by bacterial fermentation of dietary fibre and resistant starch. Enterocytes and...

Zonulin has emerged as a popular marker to assess the integrity of the intestinal mucosal barrier. Discovered by Dr Alessio Fasano, Zonulin...